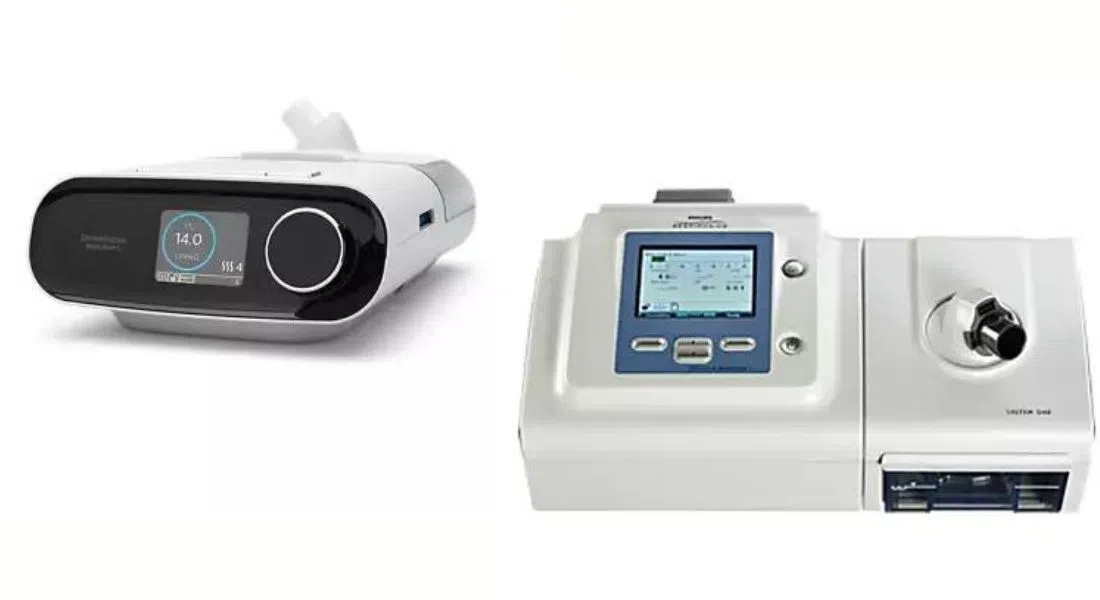
In 2021, Philips Respironics had sent out a recall for numerous models of their CPAP and bi-level PAP machines due to the degradation of noise-reducing foam found inside the device,
The particles could potentially be inhaled or swallowed by users, or release volatile organic compounds that may be inhaled, leading to negative health effects. The recall only affects models manufactured before April 26, 2021.
As a part of the company’s repair and replacement program, they are renewing their call for the public to register their affected machines on an online portal by the end of December 2024, with the goal of completing the program in Canada by the end of June 2025.
Philips indicates it will replace the foam component with a material that is not affected by this issue, or it will replace affected devices altogether.
Philips reports that they have received a low number of complaints. Some users have reported headache, upper airway irritation, cough, chest pressure, and sinus infection, but it is has not yet been determined if degraded foam particles or VOCs caused this. There is a link to the registration site below.
Philips Respironics Sleep and Respiratory Care devices | Philips

























